Erkek Lab. on Disease Epigenetics
Overview
Recent large-scale genomic studies which have been performed to understand the changes in genomic landscape in disease context, especially the ones carried out within the frame of international consortia for the molecular characterization of cancer provided very important hints for the deregulated genes/pathways in cancer. Among those, one clear theme emerging was the mutation of the genes involved in chromatin regulation. 25%-30% of the cancer driver mutations affect chromatin modifier genes, emphasizing the importance of studying chromatin regulation in disease context. In the recent years, studies performed within ENCODE and Roadmap consortia investigated the chromatin landscape in diverse cell and tissue types mainly via mapping different histone modifications. Results of these studies provided fundamental insights about how gene regulatory networks can be discovered with the use of chromatin state mappings. Nevertheless, these studies were largely restricted to normal tissue and cell types. Number of studies characterizing chromatin regulation on disease-basis is currently very limited and needs to be further extended and developed.
Research Interests
We are mainly interested in understanding how deregulation of chromatin contributes to the differential gene regulation and appearance of novel regulatory networks in cancer with a special focus in bladder cancer. Roughly 80% of bladder cancer patients are mutant for at least one gene involved in chromatin regulation, emphasizing the investigation of chromatin-level misregulation in this cancer. In addition to cancer, it is among the interests of our laboratory to identify changes that occur at the epigenetic level in the emergence of rare diseases. In this context, we aim to investigate the mechanisms that cause loss of function of chromatin modifiers, which are subunits of MLL complex, and frequently mutated both in cancer and in rare disease. Our ultimate goal is to comprehensively describe the changes that occur at the epigenetic level and to identify new molecular mechanisms that can be used to develop personalized diagnosis and treatment methods in diseases.
To answer our research questions, we generate diverse omics datasets at the transcriptome, epigenome and proteome level and analyze the generated data using integrative computational biology approaches. In addition, we support our results with functional experiments using molecular biology, cell biology techniques and modulating gene expression/regulation. All these analyzes are expected to comprehensively reveal the mechanisms of chromatin deregulation and genome/transcriptome regulation.
Group Members

Erkek Lab. on Disease Epigenetics

Gülden ÖZDEN YILMAZ
Researcher
gulden.ozden@ibg.edu.tr

EZGİ BOYVATLI
PhD Student
ezgi.boyvatli@ibg.edu.tr

Ahmet Can TÜLÜ
MSc Student
ahmetcan.tulu@std.ibg.edu.tr

Sude Yaren ÖZTÜRK
Undergraduate Student
yaren.ozturk@std.ibg.edu.tr
Former Members

Gizem ÖZGÜN
PhD Student
gizem.ozgun@ibg.edu.tr

Burcu AKMAN
Post-Doc Researcher
burcu.akman@ibg.edu.tr
0 (232) 299 _ _
Selected Publications
Akman B, Bursalı A, Gürses M, Suner A, Karakülah G, Mungan U, Yörükoğlu K, Erkek-Ozhan S. Nucleocytoplasmic β-catenin expression contributes to neuroendocrine differentiation in muscle invasive bladder cancer.. Cancer science. 2024 July . doi:10.1111/cas.16275.
Ozgun G, Yaras T, Akman B, Özden-Yılmaz G, Landman N, Karakülah G, van Lohuizen M, Senturk S, Erkek-Ozhan S. Retinoids and EZH2 inhibitors cooperate to orchestrate anti-oncogenic effects on bladder cancer cells.. Cancer gene therapy. 2024 April ; 31 (4) : 537-551. doi:10.1038/s41417-024-00725-3.
Guneri-Sozeri PY, Özden-Yılmaz G, Kisim A, Cakiroglu E, Eray A, Uzuner H, Karakülah G, Pesen-Okvur D, Senturk S, Erkek-Ozhan S. FLI1 and FRA1 transcription factors drive the transcriptional regulatory networks characterizing muscle invasive bladder cancer.. Communications biology. 2023 February ; 6 (1) : 199. doi:10.1038/s42003-023-04561-3.
Özden-Yılmaz G, Savas B, Bursalı A, Eray A, Arıbaş A, Senturk S, Karaca E, Karakülah G, Erkek-Ozhan S. Differential Occupancy and Regulatory Interactions of KDM6A in Bladder Cell Lines.. Cells. 2023 March ; 12 (6) : 836. doi:10.3390/cells12060836.
Guneri-Sozeri PY, Erkek-Ozhan S. Identification of the gene expression changes and gene regulatory aspects in ELF3 mutant bladder cancer.. Molecular biology reports. 2022 April ; 49 (4) : 3135-3147. doi:10.1007/s11033-022-07145-2.
Eray A, Erkek-Özhan S. Classification of bladder cancer cell lines according to regulon activity.. Turkish journal of biology = Turk biyoloji dergisi. 2021 December ; 45 (6) : 656-666. doi:10.3906/biy-2107-72.
Ozgun G, Senturk S, Erkek-Ozhan S. Retinoic acid signaling and bladder cancer: Epigenetic deregulation, therapy and beyond.. International journal of cancer. 2020 October . doi:10.1002/ijc.33374.
Eray A, Güneri PY, Yılmaz GÖ, Karakülah G, Erkek-Ozhan S. Analysis of open chromatin regions in bladder cancer links β-catenin mutations and Wnt signaling with neuronal subtype of bladder cancer.. Scientific Reports. 2020 October ; 10 (1) : 18667. doi:10.1038/s41598-020-75688-0.
Erkek S, Johann PD, Finetti MA, Drosos Y, Chou HC, Zapatka M, Sturm D, Jones DTW, Korshunov A, Rhyzova M, Wolf S, Mallm JP, Beck K, Witt O, Kulozik AE, Frühwald MC, Northcott PA, Korbel JO, Lichter P, Eils R, Gajjar A, Roberts CWM, Williamson D, Hasselblatt M, Chavez L, Pfister SM, Kool M. Comprehensive Analysis of Chromatin States in Atypical Teratoid/Rhabdoid Tumor Identifies Diverging Roles for SWI/SNF and Polycomb in Gene Regulation.. Cancer cell. 2019 January ; 35 (1) : 95-110.e8. doi:10.1016/j.ccell.2018.11.014.
Isabel TegederKatharina ThielSerap ErkekPascal D. JohannJohannes BerlandiVenu ThatikondaMichael C. FrühwaldMarcel KoolAstrid JeibmannMartin Hasselblatt. Functional relevance of genes predicted to be affected by epigenetic alterations in atypical teratoid/rhabdoid tumors. Journal of Neuro-Oncology. 2018 ; 141 (1) : 43-55.
Merk DJ, Ohli J, Merk ND, Thatikonda V, Morrissy S, Schoof M, Schmid SN, Harrison L, Filser S, Ahlfeld J, Erkek S, Raithatha K, Andreska T, Weißhaar M, Launspach M, Neumann JE, Shakarami M, Plenker D, Marra MA, Li Y, Mungall AJ, Moore RA, Ma Y, Jones SJM, Lutz B, Ertl-Wagner B, Rossi A, Wagener R, Siebert R, Jung A, Eberhart CG, Lach B, Sendtner M, Pfister SM, Taylor MD, Chavez L, Kool M, Schüller U. Opposing Effects of CREBBP Mutations Govern the Phenotype of Rubinstein-Taybi Syndrome and Adult SHH Medulloblastoma. Developmental Cell. 2018 March ; 44 (6) : 709-724. doi:10.1016/j.devcel.2018.02.012.
Weischenfeldt J, Dubash T, Drainas AP, Mardin BR, Chen Y, Stütz AM, Waszak SM, Bosco G, Halvorsen AR, Raeder B, Efthymiopoulos T, Erkek S, Siegl C, Brenner H, Brustugun OT, Dieter SM, Northcott PA, Petersen I, Pfister SM, Schneider M, Solberg SK, Thunissen E, Weichert W, Zichner T, Thomas R, Peifer M, Helland A, Ball CR, Jechlinger M, Sotillo R, Glimm H, Korbel JO. Pan-cancer analysis of somatic copy-number alterations implicates IRS4 and IGF2 in enhancer hijacking. Nature Genetics. 2018 January ; 49 (1) : 65-74. doi:10.1038/ng.3722.
Susanne N. Gröbner, Barbara C. Worst[…]Stefan M. Pfister. The landscape of genomic alterations across childhood cancers. Nature. 2018 March ; 555 (7696) : 321-327. doi:10.1038/nature25480.
. Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature. 2018 January ; 553 (7686) : 101-105. doi:10.1038/nature25169.
Paul A. Northcott, Ivo Buchhalter[…]Peter Lichter. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017 July ; 547 : 311-317.
Johann PD, Erkek S, Zapatka M, Kerl K, Buchhalter I, Hovestadt V, Jones DTW, Sturm D, Hermann C, Segura Wang M, Korshunov A, Rhyzova M, Gröbner S, Brabetz S, Chavez L, Bens S, Gröschel S, Kratochwil F, Wittmann A, Sieber L, Geörg C, Wolf S, Beck K, Oyen F, Capper D, van Sluis P, Volckmann R, Koster J, Versteeg R, von Deimling A, Milde T, Witt O, Kulozik AE, Ebinger M, Shalaby T, Grotzer M, Sumerauer D, Zamecnik J, Mora J, Jabado N, Taylor MD, Huang A, Aronica E, Bertoni A, Radlwimmer B, Pietsch T, Schüller U, Schneppenheim R, Northcott PA, Korbel JO, Siebert R, Frühwald MC, Lichter P, Eils R, Gajjar A, Hasselblatt M, Pfister SM, Kool M. Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes.. Cancer cell. 2016 March ; 29 (3) : 379-393. doi:10.1016/j.ccell.2016.02.001.
Lin CY, Erkek S, Tong Y, Yin L, Federation AJ, Zapatka M, Haldipur P, Kawauchi D, Risch T, Warnatz HJ, Worst BC, Ju B, Orr BA, Zeid R, Polaski DR, Segura-Wang M, Waszak SM, Jones DT, Kool M, Hovestadt V, Buchhalter I, Sieber L, Johann P, Chavez L, Gröschel S, Ryzhova M, Korshunov A, Chen W, Chizhikov VV, Millen KJ, Amstislavskiy V, Lehrach H, Yaspo ML, Eils R, Lichter P, Korbel JO, Pfister SM, Bradner JE, Northcott PA. Active medulloblastoma enhancers reveal subgroup-specific cellular origins.. Nature. 2016 February ; 530 (7588) : 57-62. doi:10.1038/nature16546.
Total : 17
Selected Book Chapters
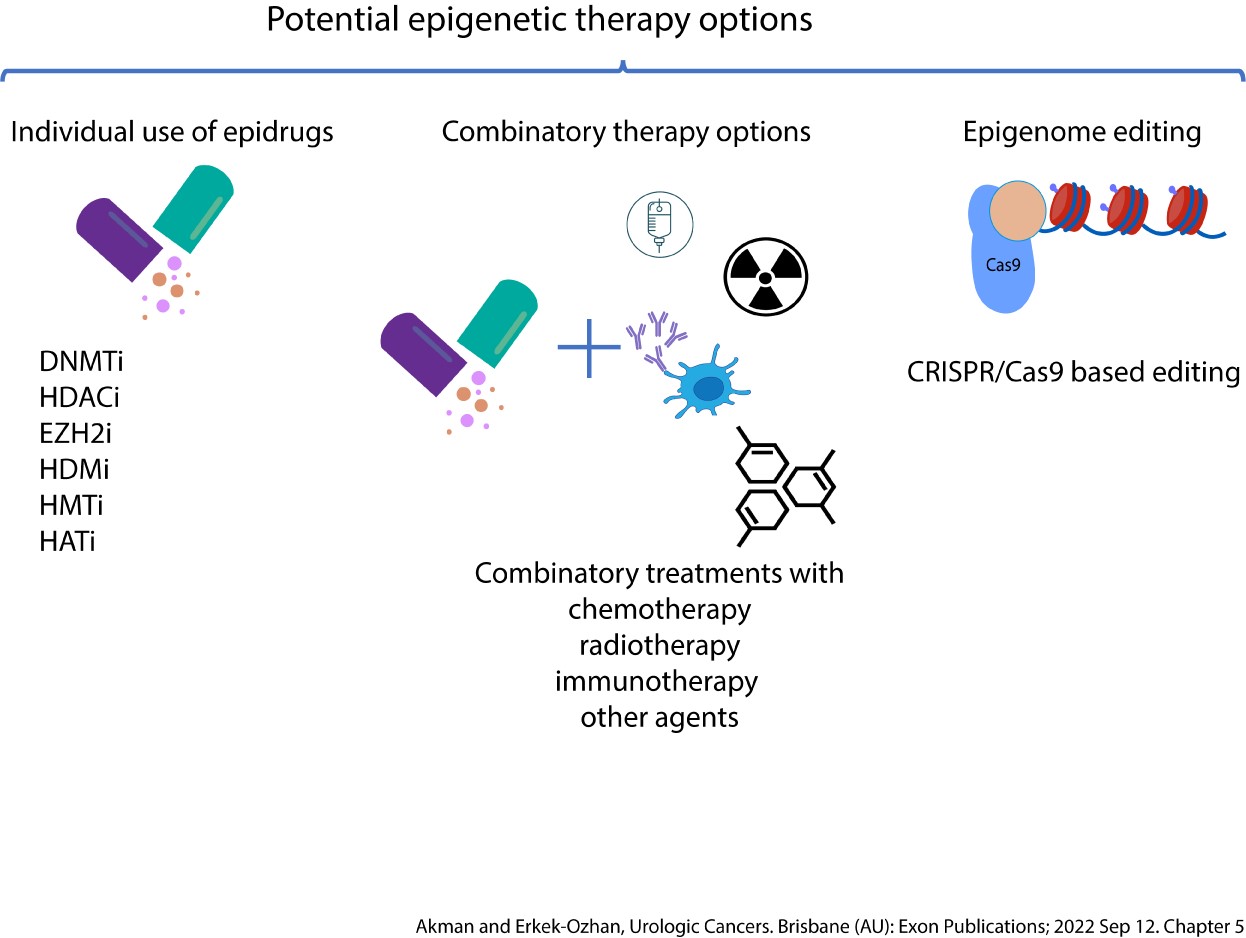
Implications of Chromatin Modifier Mutations in Epigenetic Regulation of Bladder Cancer (2022). Urologic Cancers. Exon.
Total : 1
Awards
- TUBITAK Incentive Award by TUBITAK, 2023
- International Rising Talent by L'Oréal UNESCO, 2020
- Young Scientist (BAGEP) Award by Science Academy, 2019
- Loreal UNESCO For Women in Science National Fellowship by L'Oréal UNESCO, 2019
Academic Memberships
- Tıp Bilişim Derneği, 2018
- International Bladder Cancer Network, 2019
Contact

Erkek Lab. on Disease Epigenetics