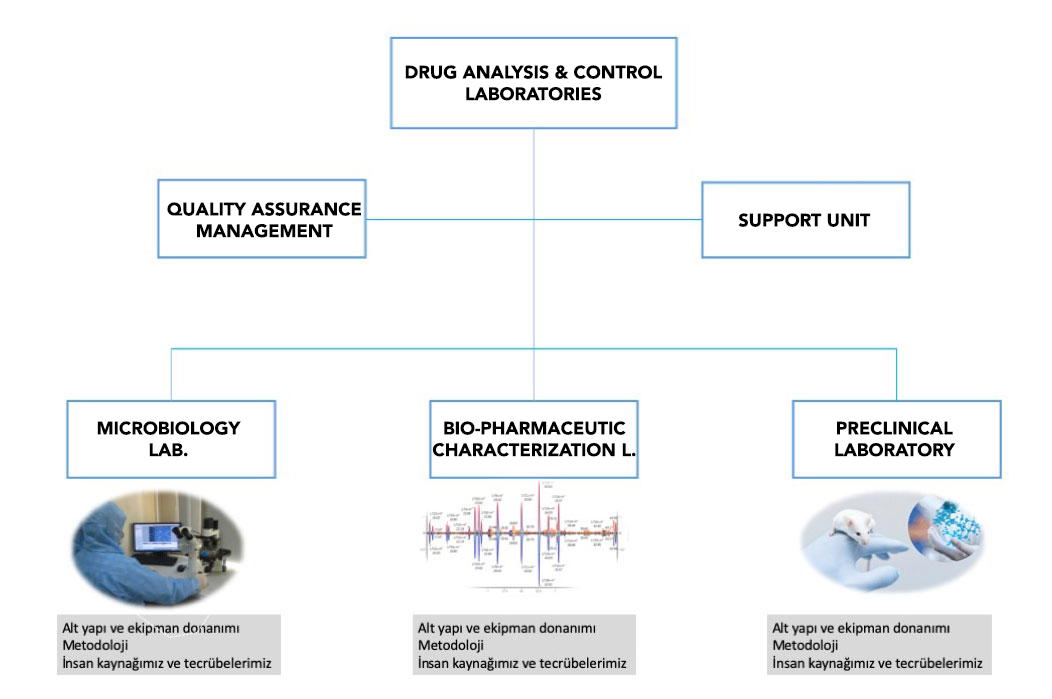
Drug Analysis and Control Lab.
DRUG ANALYSIS AND CONTROL LABORATORY
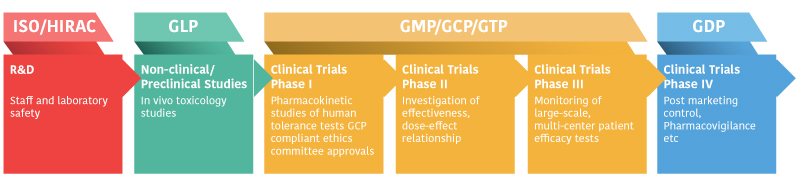
Drug development requires preclinical studies prior to IND application which should be performed in GLP certified laboratories. There is no GLP certified preclinical testing facility in Turkey, which constitutes a significant barrier for new molecular entity development. IBG has established Drug Analysis and Control Laboratories which are in compliance with GLP regulations and Quality Management System.
.jpg)
Good Laboratory Practice (GLP) in an internationally recognized indicator of technical competence in pre-clinical studies. By providing official recognition of the competence of laboratories, it ensures a convenient method for identifying and selecting reliable test and analysis services for institutions in need of preclinical work for their molecules.
Click here for contact procedure with sponsors.
Click here for the procedure including test material accepting criteria.
You can contact us directly for the services you request from IBG Drug Analysis & Control Lab.
Personnel

Drug Analysis and Control Lab.
Test Unit Manager
Ayşegül DEMİRTAŞ
aysegul.demirtas@ibg.edu.tr
+90 232 299 41 00
(2401)
+9
0 232 299 41 44

Batuhan GÜNEŞ
Technician
batuhan.gunes@ibg.edu.tr

Ezgi ERBAY
Researcher
ezgi.erbay@ibg.edu.tr

Yaren ÖZ
Veterinarian
yaren.oz@ibg.edu.tr
0 (232) 299 _ _

Hüsniye Burcu KARA
Technician
burcu.kara@ibg.edu.tr

Hanım GULA
Care Taker
hanim.gula@ibg.edu.tr

Sezer ARAZ
Cleaning Personnel
sezer.araz@ibg.edu.tr

Hamide KAYTAZ
Care Taker
hamide.kaytaz@ibg.edu.tr

Ulaş SAÇINTI
Technician
ulas.sacinti@ibg.edu.tr

Günay ATILKAN
Cleaning Personnel
gunay.atilkan@ibg.edu.tr

Serap BİRİNCİOĞLU
Advisor
serap.birincioglu@ibg.edu.tr

Serhat ÖZGENÇ
Veterinarian
serhat.ozgenc@ibg.edu.tr

Ceren ÜLKER
Veterinarian
ceren.ulker@ibg.edu.tr

Ezgi ERBAY
Quality Assurance
Department Manager
ezgi.erbay@ibg.edu.tr

Selin YEŞİL
Microbiology Laboratory
Unit Responsible
selin.yesil@ibg.edu.tr

Güzide İdil AZBAZDAR
Biopharmaceuticals Laboratory
Research Technician
idil.tilmensagir@ibg.edu.tr

Kenan AKPINAR
Cell Culture
Researcher
kenan.akpinar@ibg.edu.tr

Güneş TOK
Cell Culture
Researcher
gunes.tok@ibg.edu.tr
Former Personnel

Soner GÜNDEMİR
Platform Director
soner.gundemir@ibg.edu.tr
+90 232 299 41
00
(4201)
0 (232) 299 41 24

Mehmet İNAN
Platform Director
mehmet.inan@ibg.edu.tr
+90 232 299 41
00
(5271 - 1004)
02322994177

Alev TAŞÇIOĞLU ALİYEV
Test Unit Manager
alev.aliyev@ibg.edu.tr
+90 232 299 41
00
(2902)

Tuba KARAMAN ODUNCU
Research Technician
tuba.karaman@ibg.edu.tr

Hilmi ORHAN
Advisor
hilmi.orhan@ibg.edu.tr
+90 232 299 41
00
(3901)
02322994139

Ayşegül DEMİRTAŞ
Quality Assurance
Department Manager
aysegul.demirtas@ibg.edu.tr
+90 232 299 41 00
(2401)
0 232 299 41 44

Ezgi ERBAY
Quality Assurance
Technician
ezgi.erbay@ibg.edu.tr

Alev TAŞÇIOĞLU ALİYEV
Support
Unit Responsible
alev.aliyev@ibg.edu.tr
+90 232 299 41 00
(2902)

Berrak SOHTORİK
Support
Archieve and Documentation
berrak.sohtorik@ibg.edu.tr
+90 232 299 41 00
(3801)

Melda Zeynep GÜRAY TAŞKINARDA
Biopharmaceuticals Laboratory
Unit Responsible
melda.guray@ibg.edu.tr
+90 232 299 41 00
(2901)

Gözde ATASEVEN
Biopharmaceuticals Laboratory
Researcher
gozde.ataseven@ibg.edu.tr

Ayşegül BİLDİK
Preclinical Laboratory
Advisor
aysegul.bildik@ibg.edu.tr

Hamdi Avcı
Preclinical Laboratory
Advisor
hamdi.avci@ibg.edu.tr

Ahmet AYDOĞAN
Preclinical Laboratory
Advisor
ahmet.aydogan@ibg.edu.tr
Contact

Drug Analysis and Control Lab.
Test Unit Manager
Ayşegül DEMİRTAŞ
aysegul.demirtas@ibg.edu.tr
+90 232 299 41 00
(2401)
+9
0 232 299 41 44