
Electron Microscopy Unit
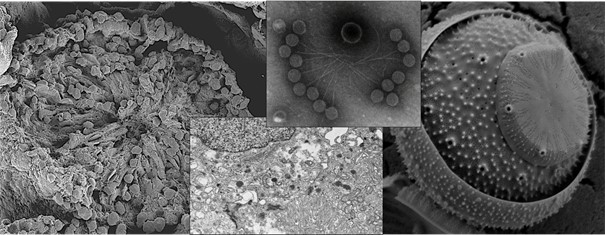
Our Electron Microscopy Laboratory is equipped with a high-resolution Field Emission Scanning Electron Microscope (FE-SEM) infrastructure. The Zeiss Sigma 500 FE-SEM system operates within an accelerating voltage range of 20 V to 30 kV and provides a resolution of 0.8 nm at 15 kV and 1.6 nm at 1 kV. Owing to its field emission electron gun, the system generates a finer and more intense electron beam compared to conventional SEM instruments, enabling superior high-resolution imaging at the nanometer scale.
The integrated EDS (Energy Dispersive X-ray Spectroscopy) detector allows elemental composition analysis of samples, including semi-quantitative elemental distribution and mapping studies. The EBSD (Electron Backscatter Diffraction) detector enables crystallographic orientation, grain structure, and phase analyses, while the STEM (Scanning Transmission Electron Microscopy) detector provides high-resolution imaging of the internal structures of biological specimens, thin films, and polymeric materials.
The Laboratory also conducts Correlative Light and Electron Microscopy (CLEM) studies. Within this approach, the same cell, tissue region, or material area is first imaged by fluorescence microscopy, followed by ultrastructural analysis of the selected regions of interest using electron microscopy. Functional and molecular data obtained by fluorescence microscopy are correlated with ultrastructural information acquired via SEM and STEM detectors, allowing precise identification of the ultrastructural counterparts of specific proteins, cellular components, or functional regions.
The Electron Microscopy Laboratory provides advanced imaging and microstructural analysis services for interdisciplinary academic research. The principal services offered include:
-
Surface morphology and ultrastructural analysis of biological tissues and cell culture samples
-
Surface topography and microstructural characterization of nanomaterials, biomaterials, and engineering materials
-
Elemental composition analysis and elemental mapping (EDS)
-
Crystallographic orientation, grain structure, and phase analysis (EBSD)
-
High-resolution imaging of internal structures of thin films, polymeric materials, and biological samples (STEM)
All services are conducted under analysis conditions tailored to the specific research objectives, with strict attention to scientific reliability and reproducibility of the acquired data. The Laboratory provides comprehensive technical support for thesis studies, funded research projects, and academic publication processes.

Zeiss Sigma 500 (FESEM)
SEM Sample Preparation
Our unit is equipped with advanced sample preparation infrastructure designed to preserve the structural integrity and surface characteristics of specimens prior to SEM analysis. Within this scope, biological samples, polymeric materials, and various engineering materials are dried under controlled conditions using the Quorum K850 Critical Point Dryer (CPD) and rendered suitable for SEM imaging. The critical point drying method eliminates surface tension–related deformations that may occur during liquid–gas phase transitions, thereby preserving cellular and ultrastructural details, particularly in biological specimens. This approach minimizes collapse, shrinkage, and morphological artifacts, enabling high-resolution SEM imaging.


For electrically non-conductive or low-conductivity specimens, the Quorum Q150R S sputter coater is used to prepare samples for SEM analysis. During coating, samples are homogeneously coated with a controlled thickness of gold/palladium (Au/Pd) alloy, which enhances surface conductivity and minimizes electron beam–induced charging effects. Consequently, image stability, signal quality, and contrast are significantly improved. Coating parameters are optimized according to specimen type and intended analytical conditions.
Sample preparation workflows are planned based on the type of analysis to be performed (morphological, elemental, crystallographic, etc.) and imaging parameters, allowing flexible, specimen-specific optimization.
TEM Sample Preparation
Sample preparation procedures for Transmission Electron Microscopy (TEM) analyses are meticulously planned and conducted to preserve the ultrastructural integrity of specimens. Biological tissue and cell samples are subjected to appropriate primary fixation, post-fixation, and tissue processing steps, followed by embedding in epoxy resin (Epon).
Using the Leica EM TRIM2 trimming system, Epon blocks are prepared for sectioning, and semi-thin and ultrathin sections are obtained with the Leica EM UC7 ultramicrotome. Semi-thin sections are used for light microscopic pre-evaluation and localization of regions of interest, while ultrathin sections are prepared for electron microscopy analyses and mounted on suitable grids.
Ultrathin sections are imaged using the STEM detector integrated into the FE-SEM system at our center. This configuration enables high-resolution STEM-mode imaging of biological specimens, thin films, and polymeric materials. The FE-SEM–STEM approach provides detailed and high-contrast visualization of internal structures, particularly in thin sections.


In addition to solid samples, specimens in solution can also be analyzed using the STEM detector. Such samples are deposited onto appropriate grids and prepared by negative staining or adsorption-based methods, allowing detailed morphological evaluation of protein complexes, vesicles, virus-like particles, nanoparticles, and polymer suspensions.
TEM sample preparation and sectioning workflows are conducted to provide comprehensive technical infrastructure support for thesis studies, funded research projects, and advanced scientific analyses.
Publications Contributed By Our Unit
- Demir Ş, Erdal E, Bagriyanik HA. Imaging of Isolated Exosomes by Correlative Microscopy. Journal of Histochemistry & Cytochemistry. 2024;72(3):149-156. 10.1369/00221554241233346
- Turker-Burhan M, Ersoy N, Bagriyanik HA, Tozburun S. Guide mapping for effective superficial photothermal coagulation of the esophagus using computer simulations with ex vivo sheep model validation study. Lasers Surg Med. 2022;54:1116–1129. https://doi.org/10.1002/lsm.23595
- Lobban CS, Bizsel N, Blanco S. Rimoportula Distribution, Heterovalvy and Heteropolarity in Hyalosira (Bacillariophyta: Rhabdonematales), with Revision of H. hesperia and Two New Species. Protist 2022;173:125869. https://doi.org/10.1016/J.PROTIS.2022.125869.
- Lobban CS, Majewska R, Ashworth M, Bizsel N, Bosak S, Kooistra WHCF, et al. Diatom Genus Hyalosira (Rhabdonematales emend.) and Resolution of its Polyphyly in Grammatophoraceae and Rhabdonemataceae with a New Genus, Placosira, and Five New Hyalosira Species. Protist 2021;172:125816. https://doi.org/10.1016/J.PROTIS.2021.125816.
Unit Coordinator: Gökçen BİLİCİ GÜLER
