
Histopathology Unit

The Histopathology Unit is a key research infrastructure that provides qualified support to basic, translational, and clinical research through tissue-level morphological analyses. Equipped with advanced tissue processing and analysis instrumentation, the Unit offers researchers reliable, rapid, and high-quality histopathological evaluation services.
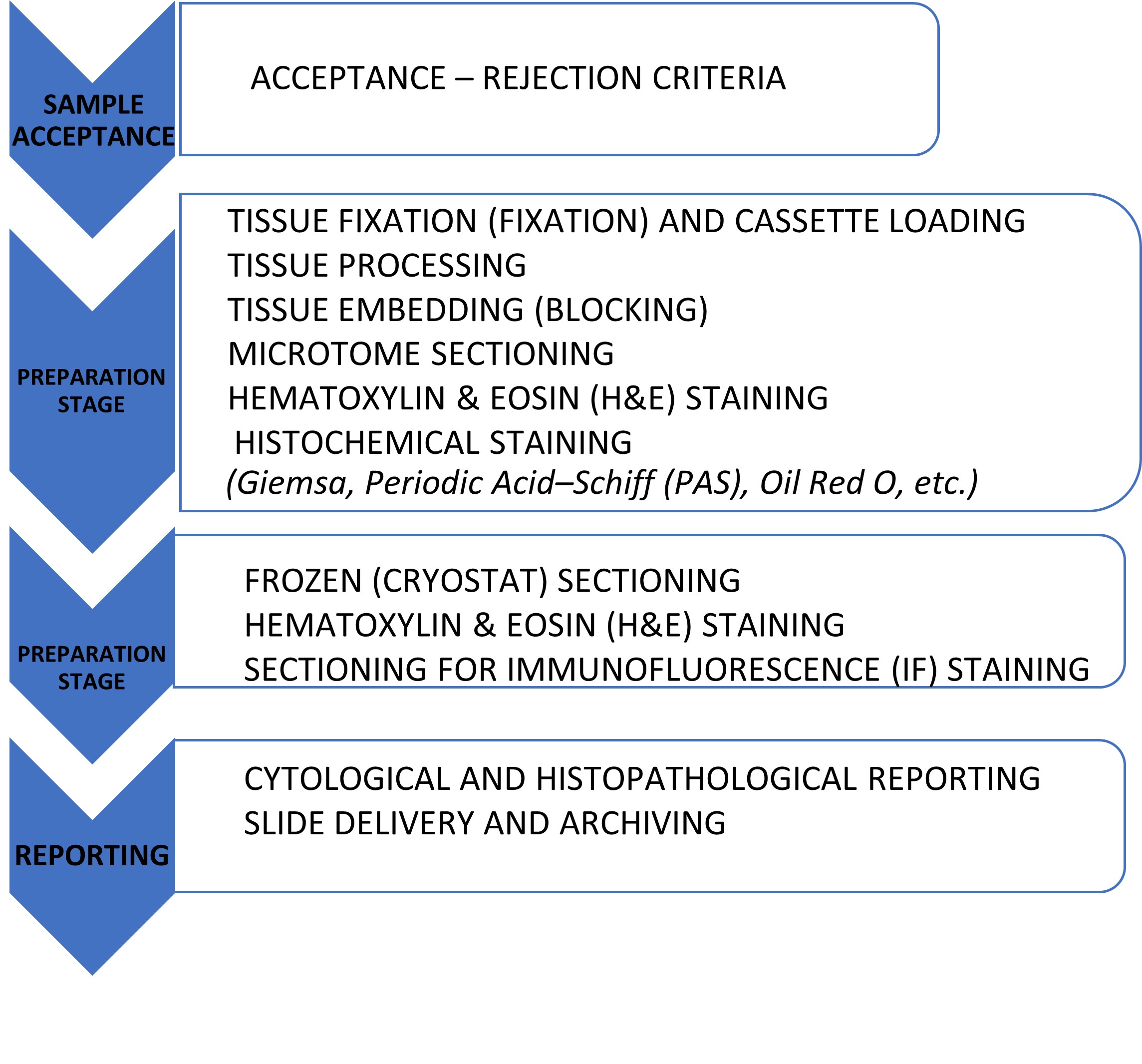
Histopathological analyses play a critical role in numerous research areas, including elucidation of disease mechanisms, evaluation of drug efficacy and toxicity, validation of biomarkers, and characterization of tissue-level pathological alterations. In this context, the Unit performs routine Hematoxylin–Eosin (H&E) staining as well as special histochemical staining procedures such as PAS and Masson’s Trichrome.
Services are provided not only to internal users but also to external academic institutions and private sector partners, with the same level of rigor, turnaround time, and quality standards.
Instruments and Equipment
- Automated tissue processor
- Paraffin tissue embedding system
- Microtome sectioning system
- Automated tissue staining and coverslipping systems
- Trinocular BX53 light microscope and microphotography systems
- Cryostat sectioning system
- Histopathology reporting infrastructure
- Cytocentrifuge
Publications Contributed By Our Unit
- Koc AC, Sari V, Kocak G, Recber T, Nemutlu E, Aberdam D, Güven S, Patient-derived cornea organoid model to study metabolomic characterization of rare disease: aniridia-associated keratopathy, BMC Ophthalmol 25, 14 (2025). https://doi.org/10.1186/s12886-024-03831-w
- Kahveci B, Polatli E, Bastanlar Y, Guven S, OrganoLabeler: A Quick and Accurate Annotation Tool for Organoid Images, ACS Omega 2024, 9, 46, 46117–46128
- Koçak G, Uyulgan S, Polatlı E, Sarı V, Kahveci B, Bursali A, Binokay L, Reçber T, Nemutlu E, Mardinoğlu A, Karakülah G, Utine CA, Güven S. Generation of Anterior Segment of the Eye Cells from hiPSCs in Microfluidic Platforms. Advanced Biology May;8(5):e2400018. doi: 10.1002/adbi.202400018. 2024
- Asal M, Kocak G, Sari V, Recber T, Nemutlu E, Utine CA, Guven S, Development of lacrimal gland organoids from human iPSC derived ocular cells, Frontiers in Cell and Developmental Biology 10, 2444, 2023
- Turker-Burhan, M., Ellidokuz, E.B., Bagriyanik, H.A. and Tozburun, S., “An endoscopic approach providing near-infrared laser-induced coagulation with accurate depth limits,” J. Biophotonics 17:e202300377 (2024).
- Turker-Burhan, M., Ersoy, N., Bagriyanik, H.A. and Tozburun, S., “Guide mapping for effective superficial photothermal coagulation of the esophagus using computer simulations with ex vivo sheep model validation study,” Lasers Surg Med. 54:1116-1129 (2022).
- Arlı, B., Dinc, O. F., T ¨ urker-Burhan, M., and Tozburun, S., “Predicting dark-field images of H&E-stained esophageal specimens,” Proc. SPIE 12630, Advances in Microscopic Imaging IV, 1263017 (2023).
- Turker-Burhan, M., Ersoy, N., Bagriyanik, H. and Tozburun, S., “Optimization study of parameters for laser-induced thermal treatment of the esophageal mucosal layer,” Proc. SPIE 12627, Translational Biophotonics: Diagnostics and Therapeutics III, 1262728 (2023).
- Turker-Burhan, M., and Tozburun, S., “An endoscopic approach to limit the depth of laser-induced thermal injury,” Proc. SPIE 12627, Translational Biophotonics: Diagnostics and Therapeutics III, 126271W (2023).
- Turker-Burhan, M., Ba˘ grıyanık, A. and Tozburun, S., “Ex-vivo Model Experimental and Simulation Results Suggesting Effective and Superficial Mucosal Photothermal Ablation at 1505 nm,” European Conferences on Biomedical Optics (ECBO) 2021, OSA Technical Digest. Paper ETh2A.8 (2021).
Unit Coordinator: Ece UZUN
